Hydroxide
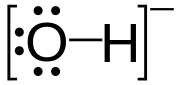
In chemistry, hydroxide is the name for the diatomic anion OH−, consisting of covalently bonded oxygen and hydrogen atoms, usually derived from the dissociation of a base. It is one of the simplest and most pervasive diatomic ions known.
Inorganic compounds that contain the hydroxyl group are referred to as hydroxides. Alkali metal (Li+, Na+, K+) and alkaline earth (Mg2+, Ca2+, Ba2+) salts containing hydroxide are common bases. Common hydroxides include
- Sodium hydroxide, NaOH
- Potassium hydroxide, KOH
- Calcium hydroxide, Ca(OH)2
Contents |
Chemical properties
When hydroxide ion is mixed with hydrogen gas, water forms. This is why water has a neutral acidity. The H+ ion cancels the basic nature of the OH- ion.
Hydroxide as a base
When hydroxides are used as bases, they usually react with hydrogen ions in the acid and form water.
HCl + NaOH → NaCl + H2O
Hydroxide as nucleophile
Hydroxide is also a strong nucleophile, converting halides to hydroxides. Characteristically hydroxides absorb carbon dioxide to give carbonates and bicarbonates:
- NaOH + CO2 → NaHCO3
Esters are saponified (converted into soap-like products) by the action of hydroxides:
- NaOH + RCO2R' → RCO2Na + R'OH
As a ligand
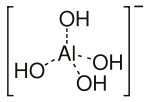
aluminate ion
The hydroxide ion is a kind of ligand. It donates lone pairs of electrons, behaving as a Lewis base. Examples of complexes containing such a ligand include the aluminate ion [Al(OH)4]− and aurate ion [Au(OH)4]−.
Solubility
Many inorganic hydroxide salts are insoluble in water. Exceptions include hydroxide salts of the Group I, Ba2+, Sr2+, Ca2+ (slightly) or Tl+.
Applications
Hydroxides and hydroxide ions are extremely useful and are the coproduct in the large chlor-alkali process. Sodium hydroxide (lye) and potassium hydroxide are particularly large scale intermediates. Many hydroxides are useful minerals such as the aluminium ore bauxite is composed largely of aluminium hydroxides. Goethite and limonite are low grade brown iron ores.
Hydrated hydroxide ion H3O2−
The mono-hydrated hydroxide ion, H3O2−, the bihydroxide ion, has been found in a small number of compounds, such as the salt Na2[N(C2H5)3CH3][Cr(PhC(S)=N-(O))3]·½NaH3O2·18H2O[1]. The H3O2− ion in this compound is centrosymmetric and has a very short hydrogen bond (114.5 pm) which is similar to the length in the bifluoride ion, HF2− (114 pm).
See also
Notes
- ↑ Kamal Abu-Dari, Kenneth N. Raymond, Derek P. Freyberg (1979). "The bihydroxide (H3O2-) anion. A very short, symmetric hydrogen bond". J. Am. Chem. Soc. 101: 3688–3689. doi:10.1021/ja00507a059.